China consults on second batch of priority chemicals
China is consulting until 31 May on a second batch of chemicals it has selected for ‘priority control’ because they are considered hazardous, highly bio-accumulative and pose the greatest risk to the environment and human health.
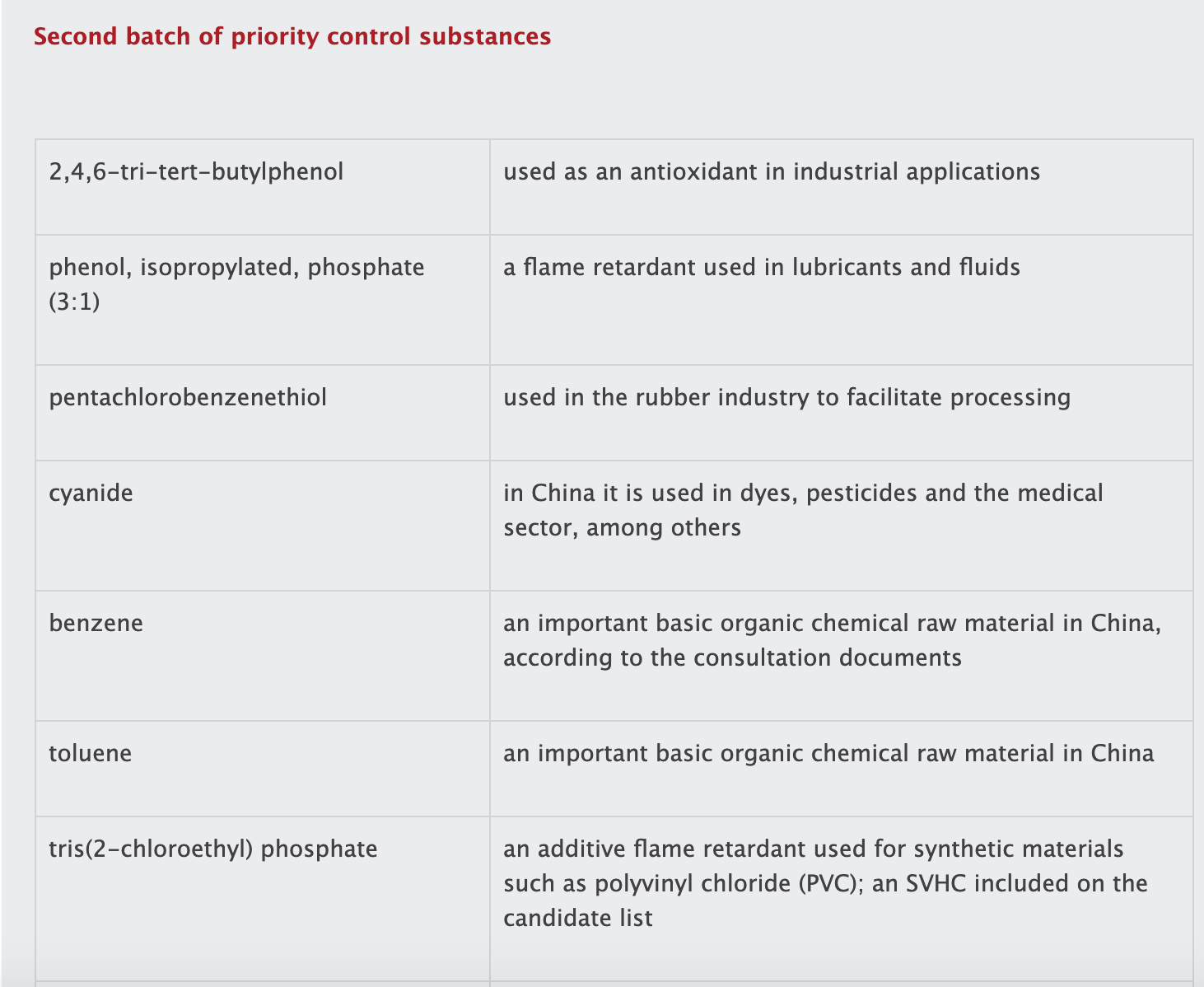
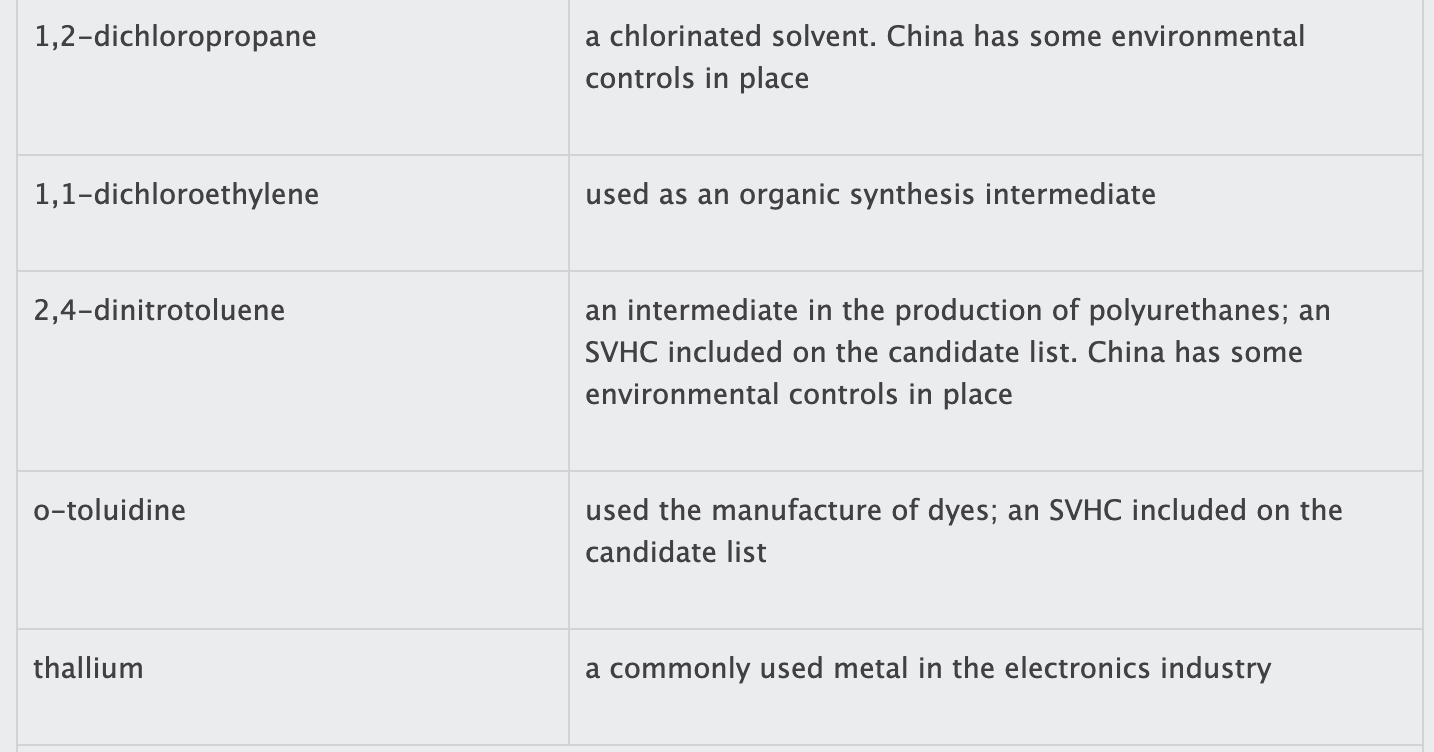
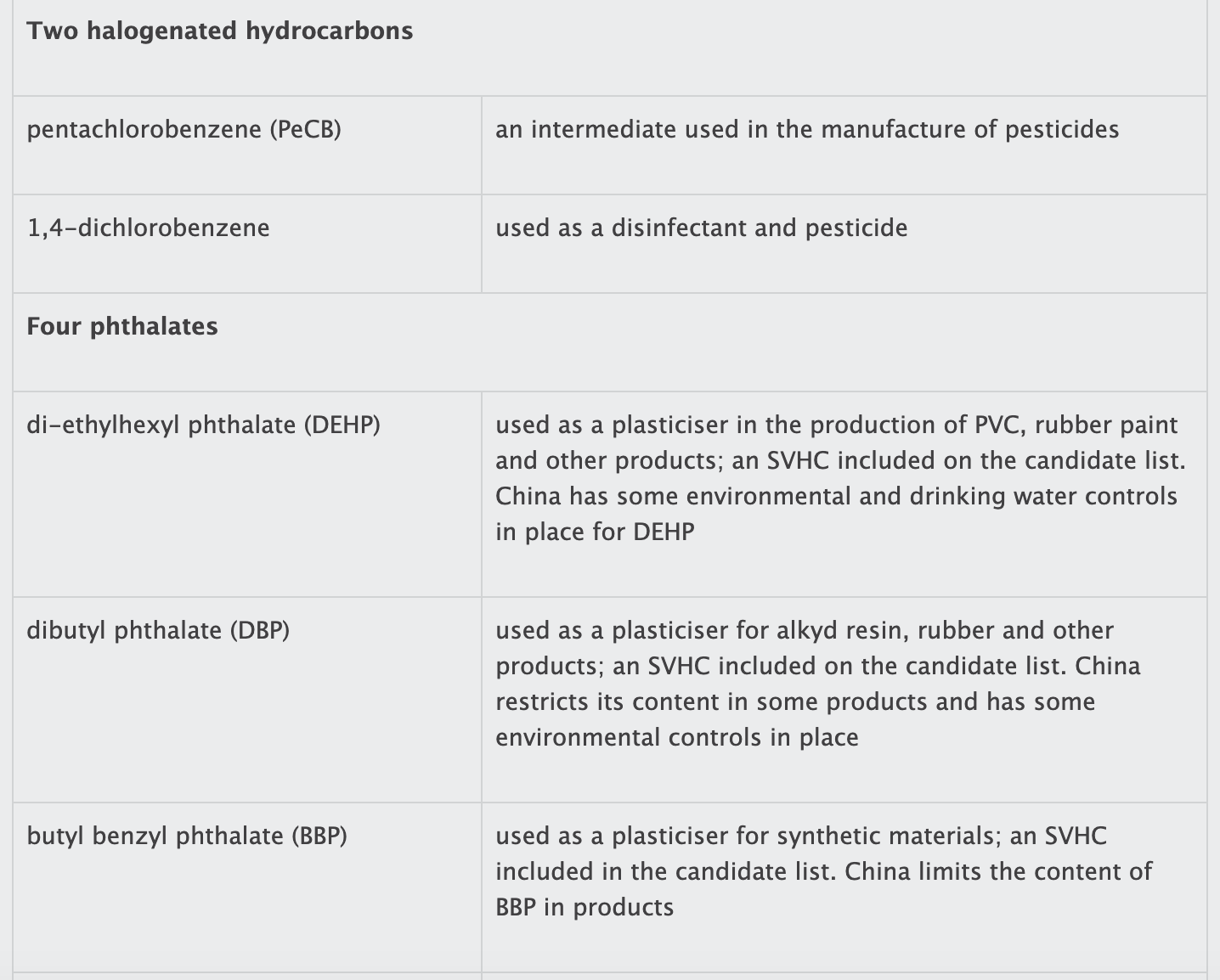
The list contains 19 substances or groups of substances (see box). While most of the substances are controlled in other jurisdictions, several of them are not currently regulated in China.
The substances have been selected based on whether:
- it is subject to restrictions in one or more developed country;
- it is manufactured in large quantities in China or has high potential for environmental exposure, including properties of persistence, bioaccumulation and toxicity (PBT); or
- an international organisation or official agency has confirmed it to be a category 1A carcinogen, mutagen or reprotoxic (CMR) substance – but the documents do not indicate which classification system the government is using.
Heng Li, senior associate at law firm Mayer Brown told Chemical Watch that, eventually, these chemicals will be governed by the incoming Environmental Risk Assessment and Control Regulation for Chemical Substances (Erac).
Once approved, Erac – which is currently in draft – will become China’s overarching chemical framework, managing both new and existing chemical substances. The draft regulation, which will affect any company handling chemicals, is similar to TSCA in the US or Japan’s Chemical Substances Control Law (CSCL) in that it would require pre-market approval of new substances and risk evaluation of certain existing ones.
"According to policies issued a few years ago, the MEE will continue to publish additional batches of priority control chemicals," said Ms Li.
"For now there is no real legal basis for governing these substances. The MEE provides very general requirements with various measures but it is not so specific over which measures apply to which substances. At the moment it is very general," she explained.
However, the list serves as "a clear message for the future and companies should pay attention to the substances on the list," ERM’s China product stewardship expert told Chemical Watch.
The MEE (then MEP) published the first batch of 22 substances at the end of 2017, but there has been no movement to regulate them since their publication.
International assessments
The list contains three of the six persistent organic pollutants (POPs) that the Ministry of Ecology and Environment (MEE) asked companies to provide information on in October last year.
The second batch also includes six substances that the US EPA designated in December last year as high-priority substances to undergo a risk evaluation under the Toxic Substances Control Act (TSCA):
tris(2-chloroethyl) phosphate;
di-ethylhexyl phthalate (DEHP);
dibutyl phthalate (DBP);
butyl benzyl phthalate (BBP);
di-isobutyl phthalate (DIBP);
and 1,2-dichloropropane.
The US EPA is due to publish final evaluations on these substances by December 2022. The initial scope documents, which identify what uses of the chemical the EPA will evaluate, are due by 22 June this year.
The consultation was published on 7 May but signed by the MEE, in conjunction with the Ministry of Industry and Information Technology (MIIT) and the National Health Commission (NHC), on 17 April.



Related Article
- Geely's Li Shufu proposes collection of vehicle purchase tax shared by central & local governments
- Shanghai natural rubber futures open higher on May 14
- Double Coin Tire post sales decline in Q1 2020
- Techking Tires delivered intelligent tire system
- China bans production of China Ⅴ light-duty vehicles from July 1, 2020
- Tireworld Insight: Domestic tire makers eye overseas expansion
- Tireworld Insight: Price disparity severe between China's rubber exports and imports
- Tireworld Insight: China tire exports dependent on US market performance
- Tireworld Insight: SHFE rubber expected to move in tight range in short-term
- Tireworld Insight: Rubber futures to test near-term resistance at 15,000 yuan/tonne
- Tireworld Insight: China’s tire industry on track of rapid growth